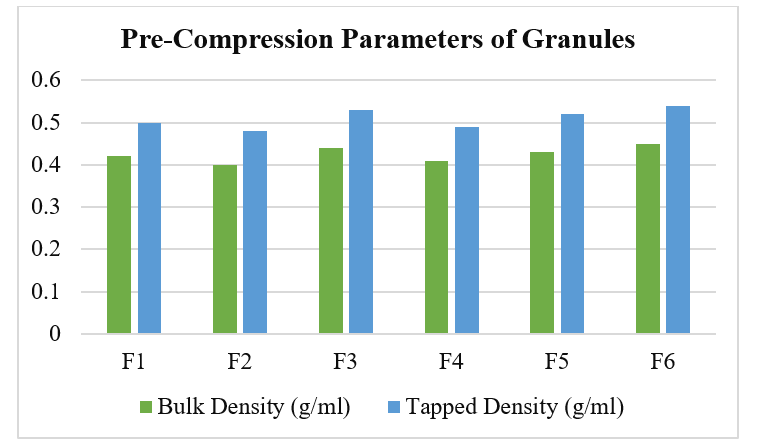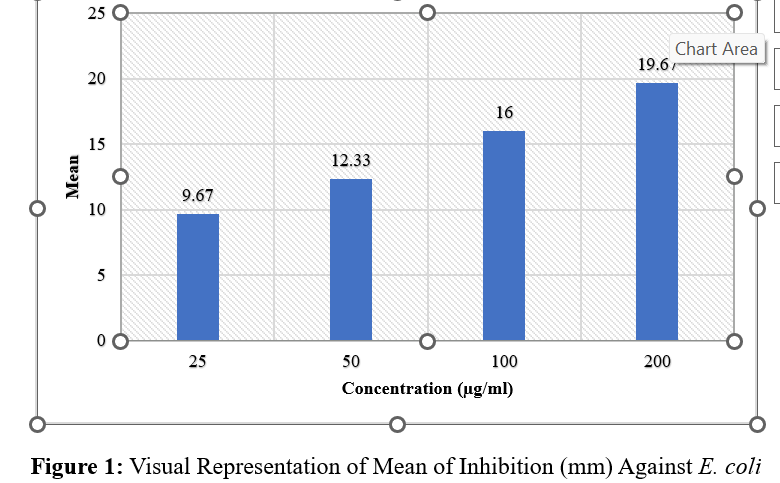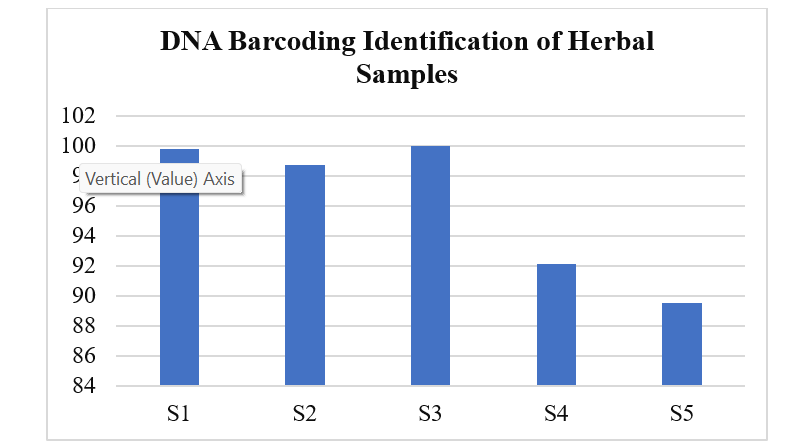
Authentication and Adulteration Detection of Herbal Raw Materials Using DNA Barcoding and FTIR Fingerprinting
The growing demand of herbal medicine in the world market has heightened the chances of adulteration and misidentification thus undermining safety, efficacy and quality. This study set out to authenticate three herbal raw materials waste components, namely, Gymnema sylvestre, Curcuma longa, and Trigonella foenum-graecum, and identify adulteration by DNA barcoding and Fourier Transform Infrared (FTIR) spectroscopy against pharmacological analysis in Wistar rats to determine biological efficacy. DNA barcoding was precise in determining is genuine species and differentiating them with adulterated samples whereas FTIR fingerprinting identified the presence of chemical adulterants and correlated with changes in therapeutic capability. Pharmacological tests identified that genuine extracts had a significant effect in lowering the level of blood glucose and had better effects than commercial and adulterated extracts, the level of which declined proportionally to the extent of adulteration. The results prove that a combination of molecular, chemical, and pharmacological techniques is a stable and successful strategy of quality control, authentication, and safety testing of herbal raw materials, and purity may play a key role in the therapeutic efficacy.